Chemistry Matters is a digital high school chemistry course offerred by Georgia Public Broadcasting. It consists of a series of 12 units of study divided into segments. Each segment provides you with support materials designed to enhance student understanding of the content, along with vocabulary and a teacher’s guide (available upon request). The Chemistry Matters teacher toolkit provides instructions and answer keys for labs, experiments, and assignments for all 12 units of study. The videos are also available on youtube.
Unit 1: Introduction to Chemistry
In this unit, students investigate a fish kill on Georgia’s Ogeechee River, and learn how to obtain, evaluate, and communicate data and information.
Segment A: What is Chemistry?
In this segment, we outline the eight science and engineering practices of chemistry. Students also discuss qualitative and quantitative observations.
Segment B: Hypotheses and Models
During this segment, we introduce two Science and Engineering Practices: Generating a Hypothesis and Developing a Model.
Segment C: Investigating the Problem
We explore the steps involved in creating and testing a hypothesis and introduce our third Science and Engineering Practice, planning and carrying out an investigation.
Segment D: Experimental Design
Experimental Design is explored as the terms manipulated variable, responding variable, and constant are discussed.
Segment E: Performing an Experiment
In this segment, students learn about the importance of lab safety procedures while performing an experiment testing the pH of various samples in the classroom.
Segment F: Analyzing Data
Students analyze data collected in the last segment and interpret that data by constructing an argument that explains whether the data shows that their hypothesis should be supported or rejected.
Segment G: Engaging in Argumentation
In this segment, students engage in argumentation by either rejecting or supporting their hypotheses about the fish kill. We hear from environmental lawyer Don Stack, who discusses the role of chemistry in his profession.
Unit 2: Introduction to Matter
Students explore the chemical and physical properties of matter and discover how scientific ideas are connected to each other rather than existing in isolation.
Segment A: Properties of Matter
In this segment, our students learn about physical properties of matter using the densities of pennies as a model.
Segment B: Density Lab Results and Crush Lab
Our students continue their discussion of penny densities in this segment and begin a crushing experiment to examine the different physical properties of chemicals.
Segment C: Physical Properties and Phase Change
In this segment, we continue with our exploration of physical properties, including brittleness and malleability. We also learn about phase changes and observe a demonstration on the freezing point of water.
Segment D: Phase Change Demonstrations
Dr. Adrian Elliott from the Fernbank Science Center joins us in this segment for a special interview, and our students discuss sublimation and deposition.
Segment E: Chemical Properties
During this segment, we learn the difference between chemical and physical properties, and we see a demonstration of reactivity.
Segment F: Mixtures
Homogeneous and heterogeneous mixtures are the focus of this segment as well as solutions and alloys.
Segment G: Separating Mixtures
Homogeneous and heterogeneous mixtures are the focus of this segment as well as solutions and alloys.
Segment H: Chromatography Results and Mixtures Challenge
Homogeneous and heterogeneous mixtures are the focus of this segment as well as solutions and alloys.
Segment I: Mixtures Challenge Results and Water Treatment
In this segment, our students discuss the results of their engineering design challenge and we hear from special guest Stan Brinkley, with the Cobb County Marietta Water Authority.
Unit 3: Atomic Structure
Students analyze conceptual, mathematical, and physical models of atoms. This unit also includes an overview of protons, neutrons, and electrons, as well as a periodic table of elements.
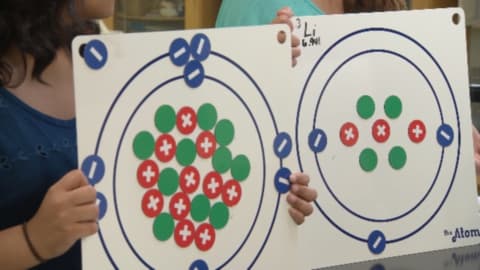
Segment A: Atomic Models
In this segment, the students learn about different models of the atom, including Dalton’s model, Thomson’s model, Rutherford’s model, and the Bohr model.
Segment B: The Periodic Table
The periodic table is the focus of this segment. The students explore periodic trends as well as atomic number and average atomic mass.
Segment C: Characteristics of Electrons
In this segment, students build models of atoms as they learn about characteristics of electrons. They also explore how quantum levels affect fireworks by performing a flame test.
Segment D: Periodic Trends Part I
Students explore the relationship between the properties of an atom and its location on the periodic table as well as about effective nuclear charge and electronegativity.
Segment E: Periodic Trends Part II
In this segment, the students make predictions about electronegativity and atomic radius across periods and columns.
Segment F: Electron Configuration Part I
This segment examines ionization energy and explains the process for writing the electron configuration of any element.
Segment G: Electron Configuration Part II
The students learn to make electron configurations in this segment. Sublevels and orbitals are also discussed.
Segment H: Configuration Lab Results and Fireworks
During this segment, special guest Mark Angeli explains the role of pyrotechnics in the making of fireworks.
Unit 4: Bonding
The focus of this unit is chemical bonds, both intermolecular and intramolecular. Students also look at the bond spectrum and learn about electrostatic and electronegative forces.
Segment A: Introduction to Bonding
This segment introduces the concept of chemical bonding and explores chemical changes using the ingredients found in taco sauce as a model.
Segment B: Chemical Bonding
In this segment, the students learn about chemical bonds, Lewis dot diagrams, and ionic bonds.
Segment C: Intramolecular Bonding
Intramolecular bonds are discussed in this segment, and the students create their own bonds using a software simulator.
Segment D: Comparing Types of Bonds
The students discuss polar and nonpolar bonds in this segment, followed by an explanation of single, double, and triple bonds.
Segment E: Intermolecular Bonding
Intermolecular forces are the focus of this segment, and the students learn why hydrogen bonding makes ice float.
Segment F: Melting Results and Molecular Modeling
The students learn about melting point using sugar and salt. Special guest Dr. Ludovice discusses molecular modeling.
Unit 5: Chemical Reactions
Students identify the primary indicators of chemical change and learn about the law of conservation of matter. This unit also covers balanced chemical equations and showing reactants and products of chemical reactions.
Segment A: Balancing Equations
The law of conservation of mass and balancing equations are the focus of this segment with students performing a lab to explore these topics.
Segment B: Types of Reactions
This segment explores different types of reactions by performing a lab – how scientists use chemical reactions to synthesize new medications, and a discussion on acid rain.
Segment C: Reactivity and Predicting Products
This segment examines reactivity and predicting the products of chemical reactions by performing labs looking at metal reactivity and solubility with double displacement reactions.
Segment D: Identifying Unknown Samples Part I
During this segment, the students perform a lab to test and identify four unknown samples.
Segment E: Identifying Unknown Samples Part II
The students discuss their flow charts and lab results, explaining how they identified their unknown substances.
Unit 6: The Mole and Stoichiometry
In this unit, students learn the mathematical tool of dimensional analysis, alternately called the factor-label method. Students also explore how to predict and measure the outcome of a reaction.
Segment A: Dimensional Analysis
Stoichiometry is introduced in this segment, and the students discuss the basics of dimensional analysis.
Segment B: The Mole
This segment examines the mole and Avagadro’s number. The students continue their exploration of dimensional analysis.
Segment C: Percent Composition and Empirical Formulas
The topics found within this segment include converting moles to liters as well as calculating percent composition, empirical formulas, and molecular formulas.
Segment D: Stoichiometric Calculations
This segment explores stoichiometric calculations and mole ratios.
Segment E: Limiting Reactants
This segment explores limiting reactants as we watch the students perform a lab with s’mores.
Segment F: Combustion Lab
In this segment, the students discuss the ratios they have calculated in a rocket combustion lab.
Segment G: Combustion Lab Results
The students analyze data from the rocket combustion lab started in the previous segment and hear from special guest Dr. David Gottfried about nanotechnology.
Unit 7: Solutions, Acids, and Bases
Students discover how solvents dissolve ionic and covalent solutes and learn how to measure solution concentration by mass percent, molarity, and molality. This unit also covers colligative properties.
Segment A: What is a Solution?
Introduction to solutions, acids and bases and their importance in chemistry. The students begin a lab, adding different salts to water at different temperatures and observing the conditions under which the salts dissolve.
Segment B: Solubility
The students learn about solubility, insolubility, and saturated solutions. They interpret the data they compiled during the lab on dissolving salts. In the classroom, the teacher tests several solutions, and they discuss the nature of chemical solutions.
Segment C: Solution Concentration
Our host describes ways to express solution concentration. The class learns about mass percent and the students propose plans for creating solutions with a known molarity.
Segment D: Dilution
The teacher and students discuss the results from the molarity lab and how to express concentrations using mass percent or molarity. The students learn how to work a dilution problem and create a plan for a lab.
Segment E: Dilution Lab Results
The students perform the dilution lab and discuss their results to understand how to make a concentrated solution more dilute using target volume and concentration, as well as accurate calculations.
Segment F: Colligative Properties
The host discusses two of the colligative properties, freezing point depression and boiling point elevation. The students make ice cream to investigate colligative properties and solve problems to find the freezing point and boiling point of different substances.
Segment G: Acids and Bases Part I
In segment G, our host introduces acids and bases, two types of solutions made of chemical compounds. In the classroom, the students and teacher investigate the properties of acids and bases and test household substances with cabbage juice to see if they are an acid or a base.
Segment H: Acids and Bases Part II
The students use litmus paper to determine the acidity of different substances and discuss the Bronsted-Lowry Model, the Arrhenius Model, and the Lewis Model. They measure the pH of different substances in water.
Segment I: Titration Lab Results and pH
The students discuss the data from their titration lab. Our host explains the importance of titration in real world applications and discusses auto ionization of water and the calculation of pH.
Segment J: pH Lab Results
The students explain how they calculated the pH of the acid used in the titration lab. The host explains how important the concepts in this unit are to chemistry. We hear an interview with Winston Eason, an agricultural extension agent with the state of Georgia.
Unit 8: Chemical Thermodynamics
This unit focuses on the three laws of thermodynamics. Students also explore endothermic and exothermic reactions and total bond energy.
Segment A: The Laws of Thermodynamics
The first segment in this unit looks at the laws of thermodynamics. The students examine heat-related phenomena of chemical thermodynamics using hot and cold packets, illustrating how thermodynamics work in the real world.
Segment B: Specific Heat
Our host explains the difference between exothermic and endothermic reactions, and our students explore the concept of specific heat capacity by predicting whether ice cubes will melt faster when placed on metal or plastic.
Segment C: Heat Transfer
Students learn about thermal conductivity and specific heat capacity and see if their predictions for how fast the ice cubes melt were correct. Our host explains how heat capacity affects the earth’s climate, and the students begin an experiment using samples of greenhouse gases.
Segment D: Greenhouse Lab Results and Calorimetry
The teacher and students discuss the data gathered from the greenhouse gases experiment from segment C. The students set up their own calorimetry experiments using polystyrene cups to make a “coffee cup calorimeter.”
Segment E: Calorimetry Lab
The students perform the coffee cup calorimetry experiment, gathering data to determine what compounds work best to create hot and cold packs.
Segment F: Calorimetry Lab Results
The students complete the design and engineering task of making hot and cold packs. They draw conclusions about what compounds they can use to create the packs based on their observations and the data they compiled.
Unit 9: Kinetics and Gases
In this unit, students learn about kinetics, which is the study of factors that affect the rate of chemical reactions. Students also investigate collision theory and the five components of kinetic molecular theory in gas.
Segment A: Reaction Rates
Introduces kinetics and looks at how molecular motion affects gases, collision theory and reaction rates. and how substances react with each other. The students design their own antacid tablet experiment to determine which conditions create the fastest rate of reaction.
Segment B: Reaction Rate Lab
Students discuss their hypotheses about altering the rates of reaction in a dissolving antacid tablet and conduct their experiments, using variables to determine if their assumptions hold true.
Segment C: Reaction Rate Lab Results and Catalysts
Students share their data and draw conclusions from the antacid tablet experiments and learn how catalysts affect reaction rates. The teacher discusses the behavior of gases at the macro and molecular level.
Segment D: Kinetic Molecular Theory
The host describes the different properties of gases and the components of Kinetic Molecular Theory. Students are asked to make predictions about what will happen during demonstrations with gases using a balloon and marshmallow, applying the concepts of ideal gas law.
Segment E: Ideal Gas Law
This segment dives further into the ideal gas law. The teacher gives the students an engineering and design challenge: create a safe model of an airbag using what they have learned about the behavior of gases.
Segment F: Air Bag Lab
Students discuss the calculations and procedures they need for the model air bag experiment and begin making their models and recording their findings.
Segment G: Air Bag Lab Results
Students review and discuss the results from their model airbag experiment. Our host speaks with Lea Merriwether, water quality technician at Georgia Aquarium, to discuss how gas levels affect water quality.
Unit 10: Introduction to Equilibrium
The focus of this unit is to help students understand the condition in which all competing influences counteract each other, resulting in a stable, balanced, or unchanging system.
Segment A: Chemical Equilibrium
Our host introduces equilibrium theories and the dynamic nature of chemical equilibrium. The students perform an activity using Legos to understand the nature of how forward and reverse reactions affect equilibrium and the amount of products and reactants in a reaction.
Segment B: The Equilibrium Constant Part I
This segment explains how to calculate the ratio of products to reactants and why this is important in the manufacture of chemicals for business. Students write equilibrium expressions and prepare to calculate equilibrium constant.
Segment C: The Equilibrium Constant Part II
In this segment, students find out if their calculation for the equilibrium constant of ammonia was correct. The teacher asks the students to list examples that illustrate why chemical equilibrium is important in everyday life.
Segment D: Le Chatelier’s Principle
The students explain their examples of real world chemical equilibrium, including in our bodies. The teacher demonstrates Le Chatelier’s principle using a solution of tea, showing how different additives will change the tea’s color and its equilibrium.
Segment E: Smog Lab
In segment E, our students conduct an experiment to see which gases produces smog, recording their observations and creating a data table.
Segment F: Smog Lab Results
The students review the results of the smog experiment, analyze the data, and draw conclusions. The teacher asks them to create models based on what they learned about temperature and equilibrium.
Segment G: How Temperature Affects Equilibrium
Our students show their completed models and discuss how temperatures affects equilibrium. A program manager at the Georgia Department of Natural Resources, joins our host to discuss how they monitor air quality.
Unit 11: Nuclear Chemistry
This unit focuses on isotopes, nuclear decay, fission, and fusion. Students learn how to identify different types of nuclear decay products and look at real-world applications of radioactivity.
Segment A: Radioactivity
Our host explains that nuclear chemistry is what happens in the nucleus of an atom. This segment also covers the nature of radioactivity and the release of energy as it occurs. The students perform an experiment with a cloud chamber and get to see vapor trails, which are evidence of nuclear decay.
Segment B: Nuclear Fission and Types of Radiation
This segment explains how nuclear fission creates new elements. Students also learn about the characteristics of the three particles that result from nuclear decay, alpha, beta, and gamma radiation, and the energy derived from nuclear fission.
Segment C: Half-Life
Students review results from nuclear decay experiments and learn how the law of the conservation of matter applies to nuclear chemistry. Students also learn what a half-life is, how it can be used to determine the age of a fossil, and how to solve a half-life problem
Segment D: Nuclear Fusion
This segment explores why some atoms emit radioactive particles, while other do not. Our students learn about the immense power of nuclear fusion, which produces the energy from our sun and sustains life, and they participate in a lab activity demonstrating a model of fusion using marshmallows.
Segment E: Real World Nuclear Chemistry
Students choose assignments in nuclear chemistry that reflect their learning styles and interests. We also visit an isotope laboratory at Georgia Power to see real world scientists at work as they measure levels of radiation in the environment.
Unit 12: Chemistry Matters Recap
Unit 12 is a recap of Units 1 through 11 and includes an overview of all the Georgia standards covered in this series.
Segment A: Introduction to Chemistry Review
Our host recaps the content in unit 1, ” Science and Engineering Practices,” which introduces students to using models, analyzing and interpreting data, computational thinking, constructing explanations, and more. We also review the studio interview with an environmental attorney about the Ogeechee River fish kill.
Segment B: Introduction to Matter Review
We recap Unit 2,” Introduction to Matter,” in which we covered crosscutting concepts that are used by scientists around the world and looked at the chemical and physical properties of matter. We watched a scientist demonstrate phase changes from solid to liquid to gas in our studio, using liquid nitrogen.
Segment C: Atomic Structure Review
Segment C reviews the information covered in Unit 3, “Atomic Structure.” Students looked at models of atoms, discussed the Periodic Table, and heard from a fireworks expert who explained how chemistry is behind the variety of colors of a fireworks display.
Segment D: Bonding Review
In this segment, we review Unit 4, “Chemical Bonds.” The unit covered the importance of evidence-based science and looked at both intra-molecular bonds that hold atoms together and intermolecular bonds, which exist between one molecule and another.
Segment E: Chemical Reactions Review
We see how students learned to recognize the signs of chemical change in this recap of Unit 5, “Chemical Reactions.” The unit also covered the Law of the Conservation of Matter, and students balanced chemical equations and learned to draw models representing those equations.
Segment F: The Mole and Stoichiometry Review
In this recap of Unit 6, “Stoichiometry,” our host reviews what students learned about using dimensional analysis to solve stoichiometric problems. Students performed calculations and also learned about limiting and excess reactants using s’mores and launching miniature rockets in our lab.
Segment G: Solutions, Acids, and Bases Review
This segment reviews Unit 7, “Solutions, Acids and Bases.” The students saw how solvents dissolve solutes and how the rate of dissolution can be changed. They measured the concentration of solutions by molarity, mass percent, and molality. The unit also covered pH scale and how to measure acidity and alkalinity.
Segment H: Chemical Thermodynamics Review
Our host recaps Unit 8, “Chemical Thermodynamics.” The unit covered the three laws of thermodynamics and introduced students to thermochemistry. Total Bond Energy was covered, as well as specific heat capacity.
Segment I: Kinetics and Gases Review
In the recap of the unit “Kinetics and Gases,” our host tells us that the unit covered Collision Theory. We see that the students performed a lab using antacid tablets to see how kinetics affect the speed of a reaction. Students also explored the unique properties of gases and Ideal Gas Law.
Segment J: Introduction to Equilibrium Review
“Chemical Equilibrium” was the topic of unit 10, which is reviewed in this segment. Our host reviews the definition of chemical equilibrium illustrated by an activity using Legos® and how to calculate the equilibrium constant. The unit also covered Le Chatelier’s Principle.
Segment K: Nuclear Chemistry Review
This segment recaps the Unit 11, “Nuclear Chemistry,” in which students learned about radioactivity, including alpha and beta radiation and gamma rays. They were introduced to nuclear fission and fusion and the energy each can produce. We also visited a Georgia Power nuclear chemistry lab, which measures radiation in the environment.
-love learning -your best ed lessons guide, Scott